
Hospitals Intervene 340B, JJ Lawsuit, HRSA
Hospitals intervene 340b jj lawsuit hrsa – Hospitals Intervene: 340B, JJ Lawsuit, HRSA – this headline alone throws you into the heart of a complex legal battle shaking up the healthcare world. We’re talking about the 340B drug pricing program, a lifeline for many hospitals serving vulnerable populations, and a recent lawsuit challenging its implementation. This legal clash involves massive implications for hospitals, patients, and the government agency overseeing it all, the Health Resources and Services Administration (HRSA).
Get ready to dive into the details of this high-stakes drama.
The 340B program itself is designed to help safety-net hospitals stretch their resources further, allowing them to provide affordable care to underserved communities. But accusations of misuse and non-compliance have led to this major lawsuit, bringing into question the program’s effectiveness and fairness. We’ll examine the specific claims, explore the legal arguments, and delve into the potential ramifications for hospitals and the patients they serve, painting a picture of the intense pressures and ethical considerations at play.
340B Program Overview
The 340B Drug Pricing Program is a vital safety net for vulnerable populations, ensuring access to affordable medications. Established in 1992 under the Public Health Service Act, it allows covered entities to purchase outpatient drugs at significantly reduced prices. This allows these entities to stretch their budgets, enabling them to provide more comprehensive care to their patients. The program’s effectiveness, however, is a subject of ongoing debate and scrutiny.
History and Purpose of the 340B Program
The 340B program originated from a recognition that safety-net hospitals and clinics serving a disproportionate number of low-income patients often faced financial challenges in providing necessary medications. The program aims to address this disparity by providing discounts on outpatient drugs, allowing these facilities to reinvest savings into patient care services, such as expanding their facilities, upgrading equipment, or hiring additional staff.
The ultimate goal is to improve access to healthcare for underserved communities.
Eligibility Criteria for 340B Program Participation
Hospitals and other healthcare providers must meet specific criteria to participate in the 340B program. These criteria are established and overseen by the Health Resources and Services Administration (HRSA). Key requirements often include serving a significant number of low-income patients, meeting specific financial requirements, and demonstrating a commitment to providing comprehensive healthcare services to the underserved. The exact criteria can be complex and vary depending on the type of participating entity.
The ongoing hospitals’ intervention in the 340B JJ lawsuit against HRSA is a huge deal, especially considering the potential implications for healthcare access. This situation becomes even more interesting given the recent news that rfk jr confirmed hhs secretary robert f kennedy jr , which could significantly shift the agency’s priorities and potentially influence the outcome of the 340B litigation.
We’ll have to watch closely to see how this plays out for hospitals and patients alike.
Compliance with these regulations is crucial for maintaining program participation.
Types of Hospitals Participating in the 340B Program
A wide range of hospitals participate in the 340B program. These include, but are not limited to, rural hospitals, disproportionate share hospitals (DSH), critical access hospitals (CAH), and free-standing cancer hospitals. Many of these hospitals serve vulnerable populations in medically underserved areas. The diversity of participating hospitals reflects the broad reach of the program’s mission to enhance access to care for those who need it most.
Financial Benefits and Potential Challenges of 340B Participation
The 340B program offers substantial financial benefits to participating hospitals. The significant discounts on drug prices can free up resources for other critical needs, ultimately improving the quality and scope of patient care. However, participation is not without challenges. Hospitals must navigate complex regulatory requirements and reporting procedures. Furthermore, disputes over 340B pricing and contract negotiations with pharmaceutical manufacturers can lead to legal battles and operational complexities.
Successfully managing the program requires careful planning, robust internal controls, and ongoing compliance efforts. The potential for increased revenue must be weighed against the administrative burden and the risk of non-compliance penalties.
HRSA’s Role and Oversight: Hospitals Intervene 340b Jj Lawsuit Hrsa
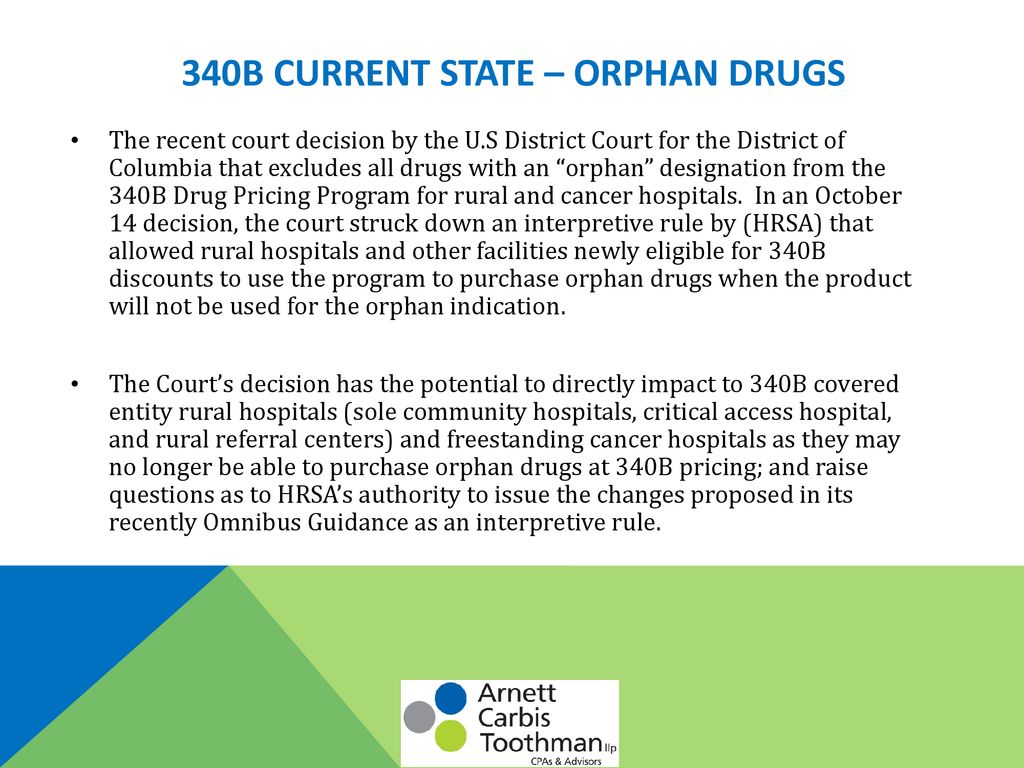
Source: slideplayer.com
The Health Resources and Services Administration (HRSA) plays a crucial role in the 340B Drug Pricing Program, acting as both the regulator and overseer. Their responsibilities extend from establishing and updating program regulations to monitoring compliance and enforcing penalties for violations. This oversight is vital to ensure the program’s integrity and its continued ability to support safety-net hospitals in providing care to underserved populations.HRSA’s responsibilities are multifaceted and significant.
They are tasked with interpreting and implementing the statutory requirements of the 340B program, clarifying ambiguities, and issuing guidance documents that help participating hospitals understand and meet their obligations. This includes defining eligible entities, outlining permissible drug dispensing practices, and specifying record-keeping requirements. Furthermore, HRSA’s role extends to investigating allegations of non-compliance, conducting audits, and ultimately imposing sanctions on those found to be in violation.
The ongoing hospitals’ intervention in the 340B JJ lawsuit against HRSA highlights the complexities of healthcare funding. This fight for drug pricing discounts makes me wonder about the impact on smaller systems; the recent news about HSHS Prevea closing Wisconsin hospitals and health centers, as reported here: hshs prevea close wisconsin hospitals health centers , raises concerns about access to care, especially given the ongoing 340B debate and its potential effects on already struggling facilities.
HRSA’s Enforcement Mechanisms, Hospitals intervene 340b jj lawsuit hrsa
HRSA employs several enforcement mechanisms to ensure compliance with 340B regulations. These mechanisms range from proactive monitoring and auditing to reactive investigations triggered by complaints or identified discrepancies. HRSA may conduct on-site audits of participating hospitals, review their 340B drug purchasing and dispensing records, and analyze their data for inconsistencies or potential violations. They may also issue warnings or demand corrective actions for minor infractions.
For more serious violations, HRSA can impose civil monetary penalties, temporarily suspend a hospital’s participation in the program, or even permanently debar them from future participation. The severity of the penalty is generally proportionate to the nature and extent of the violation.
Examples of HRSA Actions Addressing 340B Violations
HRSA has taken action against numerous hospitals for 340B violations over the years. For instance, in some cases, hospitals have been penalized for improperly dispensing 340B drugs to ineligible patients or locations. Other violations have included inadequate record-keeping, failure to properly track and report drug usage, and inaccurate reporting of patient data. The penalties imposed have varied, depending on the severity and nature of the violation, and have included financial penalties, corrective action plans, and temporary or permanent exclusion from the 340B program.
While specific details of individual cases are often confidential due to privacy concerns, publicly available information from HRSA’s website and news reports offer examples of the types of actions taken.
Recent HRSA Policy Changes and Updates
HRSA periodically updates its 340B program guidance and regulations to address emerging issues and ensure the program’s effectiveness. Recent changes have focused on clarifying ambiguities in existing regulations, strengthening enforcement mechanisms, and enhancing oversight of participating hospitals. These updates often reflect feedback received from stakeholders, including hospitals, manufacturers, and patient advocacy groups. For example, there have been recent clarifications regarding the permissible use of 340B drugs in contract pharmacies and the proper documentation required to demonstrate compliance.
Staying abreast of these changes is critical for hospitals to maintain compliance and avoid potential penalties.
The Lawsuit’s Context and Claims
The 340B drug pricing program, designed to help safety-net hospitals serve vulnerable populations, has been the subject of significant legal challenges. One such lawsuit focuses on allegations of hospital interventions and non-compliance with 340B regulations. Understanding the context and claims within this litigation is crucial for grasping the ongoing debate surrounding the program’s effectiveness and oversight.
Key Parties Involved
This lawsuit involves a complex interplay of parties. The plaintiffs are typically the pharmaceutical manufacturers or distributors who allege that participating hospitals are improperly claiming discounts under the 340B program. The defendants are the hospitals themselves, accused of violating the program’s rules and regulations. The Health Resources and Services Administration (HRSA), the federal agency responsible for overseeing the 340B program, often plays a significant, albeit indirect, role as the ultimate arbiter of program compliance and interpretation of regulations.
Individual states’ Attorneys General may also become involved depending on the scope of the alleged violations and their impact on state residents.
Plaintiffs’ Main Arguments
The plaintiffs’ arguments generally center on the assertion that hospitals are improperly expanding their 340B covered entities and locations, thereby claiming discounts on a far greater volume of drugs than permitted under the program’s guidelines. They contend that hospitals are violating the intent of the 340B program, which is intended to assist safety-net hospitals in providing care to underserved populations, not to increase profits.
This often involves accusations of exploiting loopholes in the regulations or misrepresenting their patient populations.
The ongoing legal battles surrounding hospitals, the 340B drug pricing program, and the JJ lawsuit against HRSA are complex, impacting patient care in unexpected ways. For instance, resource allocation shifts could affect access to specialized care, such as the effective strategies for managing Tourette Syndrome in children, which you can learn more about here: strategies to manage Tourette syndrome in children.
Ultimately, the outcome of the hospitals intervene 340B JJ lawsuit HRSA will significantly influence the availability of these vital services for vulnerable populations.
Allegations of Non-Compliance
Specific allegations vary from case to case, but common themes include: excessive expansion of contract pharmacies beyond what’s reasonably necessary to serve their low-income patients; improper billing practices, such as billing for drugs dispensed to patients who are not eligible for 340B discounts; and failure to maintain adequate record-keeping to demonstrate compliance with the program’s complex rules.
These allegations often involve detailed analyses of hospital billing data and patient demographics to demonstrate patterns of non-compliance.
Legal Basis for Claims
The legal basis for these lawsuits typically stems from violations of the 340B program’s regulations themselves, as well as potential violations of state and federal laws related to fraud and misrepresentation. Plaintiffs often cite specific sections of the Public Health Service Act and accompanying regulations to support their claims of non-compliance. The legal arguments hinge on proving that the hospitals’ actions constituted intentional or negligent misrepresentation to gain financial advantage through improper use of the 340B program.
Summary of Key Claims
| Claim | Evidence | Legal Basis | Potential Outcome |
|---|---|---|---|
| Improper Expansion of Contract Pharmacies | Analysis of pharmacy locations, patient demographics, and prescription data showing disproportionate dispensing compared to patient need. | Violation of 340B program regulations regarding eligible entities and locations. | Financial penalties, injunction against future improper dispensing, repayment of improperly claimed discounts. |
| Billing for Ineligible Patients | Review of patient records and billing data to identify instances where 340B discounts were claimed for patients not meeting eligibility criteria. | Violation of 340B program regulations and potential fraud charges. | Financial penalties, repayment of improperly claimed discounts, potential criminal charges. |
| Inadequate Record-Keeping | Audits demonstrating insufficient documentation to support 340B claims. | Violation of 340B program regulations requiring comprehensive record-keeping. | Financial penalties, injunction requiring improved record-keeping practices. |
| Misrepresentation of Patient Population | Analysis of patient data demonstrating discrepancies between reported low-income patient populations and actual served populations. | Violation of 340B program regulations and potential fraud charges. | Financial penalties, repayment of improperly claimed discounts, potential criminal charges. |
Impact on Hospitals and Patients
The 340B lawsuit against HRSA carries significant implications for participating hospitals and the patients who rely on the program for affordable medications. The potential financial ramifications are substantial, varying greatly depending on the hospital’s size, location, and reliance on 340B discounts. The outcome could also drastically alter access to essential medicines for vulnerable populations.Potential Financial Impact on Participating HospitalsThe lawsuit challenges the legality of HRSA’s interpretation and enforcement of 340B regulations.
A ruling against HRSA could significantly reduce the amount of drug discounts hospitals receive. For hospitals heavily reliant on 340B savings – often those serving a large proportion of low-income patients – this could lead to substantial budget cuts, potentially impacting staffing, services, and overall financial stability. Conversely, larger, wealthier hospitals might experience a less severe impact, though they too would likely see a reduction in their 340B revenue.
The extent of the financial hit will depend on the specific aspects of the ruling and the subsequent actions taken by pharmaceutical manufacturers and HRSA. For instance, if the court mandates a complete overhaul of the 340B program, some hospitals could face millions of dollars in lost revenue annually. A more limited ruling might have a less severe, but still noticeable, impact.
Consequences for Patients Relying on Discounted Medications
Reduced 340B discounts directly translate to higher medication costs for patients. For many individuals already struggling financially, even a small increase in prescription drug prices can be prohibitive, leading to medication non-adherence. This could result in worsening health outcomes, increased hospital readmissions, and a higher burden on the healthcare system as a whole. Patients relying on 340B-discounted medications in rural areas or underserved communities might be disproportionately affected due to limited access to affordable alternatives.
The potential consequences include delayed or forgone treatment, leading to potentially serious health complications. This underscores the crucial role of the 340B program in ensuring access to care for vulnerable populations.
Impact on Different Types of Hospitals
The impact of the lawsuit will vary widely depending on the hospital’s characteristics. Small, rural hospitals, often operating on tight budgets and serving a high proportion of low-income patients, are particularly vulnerable. The loss of 340B discounts could force them to make difficult choices, potentially including service cuts or even closure. Larger, urban hospitals, with more diverse revenue streams, might be less affected proportionally, although they too would experience a reduction in their revenue.
Teaching hospitals, which heavily rely on the 340B program to support their educational and research missions, would also face significant financial challenges. The disparity in impact highlights the potential for the lawsuit to exacerbate existing inequalities in healthcare access and quality.
Hypothetical Scenario: County General Hospital
Consider County General Hospital, a small, rural hospital in a low-income area. Currently, 70% of its patients rely on the 340B program for their medications. The hospital receives approximately $2 million annually in 340B discounts. If the lawsuit results in a 50% reduction in these discounts, County General would lose $1 million annually. This could force them to reduce staffing, limit services, or even consider closing its doors.
This, in turn, would leave its vulnerable patient population with severely limited access to affordable medications and potentially life-saving care. The ripple effect would be felt throughout the community, impacting not only the hospital’s patients but also its employees and the local economy.
Potential Outcomes and Implications
The 340B lawsuit against HRSA carries significant weight, potentially reshaping the landscape of hospital access to affordable medications and patient care. The legal arguments, centered around the interpretation of statutory language and regulatory actions, could lead to several distinct outcomes, each with far-reaching consequences for hospitals, patients, and the HRSA itself. Understanding these potential outcomes requires examining both the legal precedents and the potential policy ramifications.The outcome of this lawsuit hinges on the court’s interpretation of the 340B statute and HRSA’s authority to implement and enforce its regulations.
A key consideration will be whether the court finds HRSA’s actions to be within its statutory authority or an overreach. Similar past lawsuits challenging government regulations, particularly those involving healthcare programs, could serve as valuable precedents. For example, court decisions regarding Medicare and Medicaid reimbursement rates could offer insights into how courts might interpret the 340B regulations.
Additionally, cases dealing with administrative law and agency deference will play a crucial role in shaping the judge’s decision.
Potential Legal Outcomes and Their Impact
The potential outcomes of the lawsuit can be broadly categorized, each carrying unique implications. A thorough analysis of these outcomes necessitates considering their impact on hospitals, patients, and HRSA’s operational framework.
- Outcome 1: The court upholds HRSA’s regulations. This outcome would solidify the current 340B program structure and its associated regulations. Hospitals would continue to operate under the existing framework, while HRSA maintains its oversight authority. Patients would likely see no immediate change in access to discounted medications. However, this outcome might not address underlying concerns regarding program integrity or the potential for abuse.
- Outcome 2: The court partially invalidates HRSA’s regulations. This scenario involves a partial judicial overturning of specific aspects of the 340B regulations, while upholding the core program. This might lead to adjustments in program administration, potentially impacting hospitals’ ability to access certain discounts or requiring modifications to their 340B compliance procedures. Patients could experience some disruptions, depending on the specific regulations overturned. HRSA would be required to revise its regulations accordingly.
- Outcome 3: The court fully invalidates HRSA’s regulations. This is the most disruptive outcome. It would dismantle the current 340B regulatory framework, potentially creating significant uncertainty for hospitals and patients. Hospitals would lose access to the 340B discounts, potentially impacting their financial stability and their ability to provide care to vulnerable populations. Patients could face higher prescription drug costs, reducing their access to essential medications. HRSA would be tasked with either completely rewriting the 340B regulations or proposing a completely new framework.
Potential Policy Changes
Depending on the court’s decision, several policy changes could be initiated. These could include revisions to the 340B statute itself, requiring legislative action. Alternatively, HRSA might undertake a complete overhaul of its 340B regulations, potentially incorporating stronger oversight mechanisms to address concerns about program integrity. Furthermore, there might be an increased focus on developing alternative strategies to ensure access to affordable medications for vulnerable populations.
The exact nature of these policy changes will depend on the specifics of the court’s ruling and the subsequent legislative and administrative responses.
Ethical Considerations
The 340B drug pricing program, while designed to assist safety-net hospitals in serving vulnerable populations, presents complex ethical dilemmas when its implementation strays from its intended purpose. The recent lawsuit highlights potential conflicts between maximizing profits and upholding the program’s ethical mandate. Analyzing the hospitals’ actions requires careful consideration of various ethical frameworks and the potential impact on both the hospitals themselves and the patients they serve.
Potential Conflicts of Interest in 340B Program Participation
Hospitals participating in the 340B program face several potential conflicts of interest. The inherent tension lies in balancing the financial benefits derived from discounted drug pricing with the ethical obligation to provide high-quality, affordable care. For example, a hospital might prioritize enrolling patients in the 340B program to maximize its drug discounts, even if it means diverting resources away from other crucial patient services.
This prioritization could be viewed as a conflict of interest, placing financial incentives above patient well-being. Another conflict arises from the potential for hospitals to manipulate patient designations to increase their 340B reimbursements, potentially compromising the integrity of the program and the trust placed in it by government regulators. The potential for undue influence by pharmaceutical companies offering favorable deals to participating hospitals also presents a significant ethical concern.
Ethical Frameworks for Evaluating Hospital Actions
Several ethical frameworks can be applied to assess the actions of hospitals involved in the lawsuit. A utilitarian approach would focus on maximizing overall benefit and minimizing harm. This perspective would weigh the benefits of increased hospital revenue through the 340B program against the potential harm to patients who might be denied access to necessary care due to resource allocation shifts.
A deontological approach would emphasize the inherent rightness or wrongness of actions, regardless of their consequences. This perspective might criticize actions taken by hospitals that violate the spirit of the 340B program, even if those actions lead to increased financial gains. Virtue ethics would focus on the character and moral integrity of the actors involved, assessing whether their actions align with virtues such as honesty, fairness, and compassion.
Applying these frameworks reveals the multifaceted ethical complexities of the situation and highlights the lack of a single, universally accepted solution.
Ethical Responsibilities of Hospitals Participating in the 340B Program
The ethical responsibilities of hospitals participating in the 340B program are significant and far-reaching. Failing to adhere to these responsibilities undermines the program’s core purpose and erodes public trust.
- Transparency and Accountability: Hospitals should maintain complete transparency regarding their 340B program participation, including their enrollment procedures, drug acquisition and dispensing practices, and the use of resulting savings.
- Patient-Centric Approach: The primary focus should remain on providing high-quality, affordable care to vulnerable populations, not maximizing financial gains from the program.
- Compliance with Regulations: Hospitals must rigorously adhere to all HRSA regulations and guidelines governing the 340B program, avoiding any actions that could be construed as fraudulent or manipulative.
- Fair Resource Allocation: Savings generated through the 340B program should be reinvested in ways that directly benefit the underserved communities the program is designed to serve.
- Conflict of Interest Management: Hospitals must establish and maintain robust mechanisms to identify, manage, and mitigate potential conflicts of interest arising from their participation in the 340B program.
Closing Notes

Source: slideplayer.com
The Hospitals Intervene 340B lawsuit against HRSA is far from over, and its outcome will significantly impact the future of healthcare access for vulnerable populations. The legal battles, the financial stakes, and the ethical dilemmas all highlight the urgent need for a thorough review of the 340B program. This case serves as a stark reminder of the complexities inherent in balancing the need for affordable healthcare with the prevention of potential program abuse.
The decisions made now will shape healthcare policy for years to come, impacting not only hospitals but the lives of countless patients who rely on the program’s benefits.
Top FAQs
What is the 340B Drug Pricing Program?
It’s a federal program that provides discounts on outpatient drugs to eligible hospitals serving a disproportionate number of low-income patients.
Who are the key players in this lawsuit?
The lawsuit involves hospitals (both plaintiffs and defendants), pharmaceutical companies, and HRSA, the government agency overseeing the 340B program.
What are the potential consequences of the lawsuit for patients?
Depending on the outcome, patients could face higher drug costs or reduced access to medications if participating hospitals lose access to 340B discounts.
What are the ethical concerns surrounding the 340B program?
Concerns include potential conflicts of interest and ensuring that discounts reach the intended patients, not just boosting hospital profits.





